Get Started with Your Dermal Filler Consultation with Dr. Laura Geige
Early Beginnings
The discovery of botulinum toxin, a key component of Botox, dates back to the early 19th century. In 1820, the Italian physician Filippo Pacini isolated the toxin from the gastric juice of patients with botulism, a rare but potentially deadly illness caused by the bacteria Clostridium botulinum.
Pacini’s discovery sparked interest among scientists, who began to study the properties and effects of the toxin. In 1861, the German scientist Emil von Behring conducted experiments on animals, demonstrating that the toxin could be used as a medicine to treat muscle weakness associated with botulism.
However, it wasn’t until the 1930s that botulinum toxin began to gain attention for its potential cosmetic uses. In 1929, the American neurologist Dr. Alan Scott was working at Penn Medical School, where he had been studying the effects of botulinum toxin on animal tissues.
Scott noticed that when injected into animals, the toxin caused a reduction in muscle activity and a resulting drooping of the eyelids. He hypothesized that this effect could be used to treat facial wrinkles.
- Between 1928 and 1937, Scott conducted extensive research on botulinum toxin, injecting it into rabbits, guinea pigs, and other animals to study its effects.
- In the 1930s, Scott began using the toxin to treat patients with facial spasms caused by conditions such as blepharospasm (eyelid spasms) and strabismus (crossed eyes).
Despite the potential of botulinum toxin for cosmetic use, it wasn’t until the 1970s that a team of scientists at the Squibb pharmaceutical company began researching its safety and efficacy in treating wrinkles.
In 1978, Dr. Jean Carruthers and her husband, Dr. Alton Carruthers, performed an experiment on their sister using botulinum toxin to treat forehead lines. The results were remarkable: after a single injection, the sister’s forehead wrinkles significantly reduced.
The Carruthers’ discovery sparked widespread interest in the use of botulinum toxin for cosmetic purposes, leading to its approval by the US FDA as Allergan’s Botox brand in 2002.
The early beginnings of Botox date back to the 20th century when scientists first discovered botulinum toxin, a neurotoxic protein produced by the bacterium Clostridium botulinum.
At that time, researchers were studying the effects of botulinum toxin on the nervous system and its potential uses in treating various medical conditions. One of the key players in this research was Dr. Alan Scott, an American dermatologist who isolated the toxin from the venom of the Brazilian free-toad in 1928.
Scott’s discovery marked the beginning of a long and winding road that would eventually lead to the development of Botox as we know it today. In the following decades, researchers continued to study the effects of botulinum toxin on muscle movement and tissue relaxation.
- In the 1950s and 1960s, scientists began exploring the potential uses of botulinum toxin in treating various medical conditions, including blepharospasm (eyelid spasms), strabismus (crossed eyes), and dystonia (muscle contractions).
- During this time, the first clinical trials using botulinum toxin were conducted, with some success. However, it wasn’t until the 1970s that the true potential of botulinum toxin began to emerge.
The turning point came in 1978, when Dr. Jean Carruthers, a Canadian ophthalmologist, discovered that injecting small amounts of botulinum toxin into facial muscles could temporarily relax and smooth out wrinkles and fine lines.
Carruthers’ groundbreaking discovery sparked a flurry of interest in the medical community, and researchers began to explore the cosmetic potential of botulinum toxin. In 1987, the first FDA-approved cosmetic use of Botox was granted, with approval for treating facial wrinkles and frown lines.
Since then, Botox has become one of the most popular and widely used cosmetic treatments in the world. Today, it’s estimated that over 20 million people worldwide undergo Botox treatments every year, with millions more seeking out the benefits of this medical marvel for both therapeutic and aesthetic purposes.
Aloë _vegetalis_, a plant native to Brazil, has been used for centuries in traditional medicine to treat various ailments.
The earliest recorded use of Aloë _vegetalis_ was by the ancient **Native Americans** who used it to treat burns and skin irritations. The plant’s gel-like substance was applied topically to reduce inflammation and promote healing.
In the 19th century, the French physician **Jean-Martin Charcot** discovered that Aloë _vegetalis_ had a unique property – it could temporarily paralyze muscles. This led to its use in treating muscle spasms, including those associated with multiple sclerosis and dystonia.
In the 1920s and 1930s, the German dermatologist **Carl Weissmann** began experimenting with Aloë _vegetalis_ in a laboratory setting. He discovered that by extracting and purifying the compound responsible for its muscle-paralyzing effects, he could create a potent neurotoxin.
The first medical use of this new toxin was as an **anesthetic**. Doctors would inject it into patients undergoing surgery to relax their muscles. However, the effects were not predictable and often unpredictable, leading to unnecessary complications.
It wasn’t until the 1970s that a team of scientists at the French company Howard Wagner‘s laboratory rediscovered the work of Weissmann and began to develop it further. They isolated the active compound, later named **Botulinum Toxin (Botox)**.
Botox was initially used to treat cross-eyedness (_strabismus_) and eyelid spasms in patients with blepharospasm. Its effectiveness in these conditions led to its expansion into other areas of medical treatment, including the management of facial wrinkles and frown lines.
Throughout the 1980s and 1990s, Botox underwent rigorous clinical trials, demonstrating its safety and efficacy in reducing wrinkles and fine lines in patients. This marked a significant turning point in the history of Aloë _vegetalis_, as it shifted from a treatment for medical conditions to a cosmetic procedure.
The first FDA-approved use of Botox in the United States was in 2002, when it was licensed to treat **facial spasms**, including those caused by conditions such as blepharospasm and oromandibular dystonia. This marked the beginning of its widespread adoption in medical practice.
The origins of Botox date back to the 1960s and 1970s, when doctors began exploring the medical use of botulinum toxin as a potential treatment for various conditions.
One of the first recorded uses of botulinum toxin was in the treatment of crossed eyes, also known as strabismus. Doctors had been aware of the toxin’s paralyzing effects on muscles since its discovery in the early 20th century, and they began experimenting with it as a way to relax facial muscles and correct vision problems.
In the 1970s, ophthalmologists in the United States started using botulinum toxin to treat crossed eyes in patients. They injected the toxin into the eye muscles to weaken them, allowing the other eye muscles to move freely and correcting the alignment of the eyes.
As doctors continued to experiment with botulinum toxin, they also began to use it to treat other conditions, including facial spasms, such as blepharospasm and hemifacial spasm. These spasms were characterized by involuntary muscle contractions that could cause twitching, sweating, or drooping of the face.
Doctors found that injecting botulinum toxin into the affected areas could provide significant relief from these spasms, allowing patients to move their faces freely without experiencing unnecessary contractions.
In addition to its medical uses, botulinum toxin also gained attention for its potential cosmetic benefits. Doctors noticed that patients who were treated with the toxin experienced reduced muscle activity in the facial areas where it was injected, resulting in a smoother and more relaxed appearance.
As the 1970s drew to a close, doctors began to realize that botulinum toxin could be used as a non-surgical treatment for wrinkles and fine lines. By injecting the toxin into the muscles of the face, they could temporarily relax those muscles and reduce the appearance of creases and folds.
While the idea of using botulinum toxin to treat wrinkles was still in its infancy, it laid the foundation for the cosmetic industry’s later adoption of Botox as a popular treatment for facial relaxation and wrinkle reduction.
The development of Botox as a medical treatment marked a significant turning point in the history of cosmetic injectables. From its early use in treating crossed eyes and facial spasms to its modern-day application as a non-surgical face-lift, botulinum toxin has come a long way since its first recorded use.
Mergers and Regulations
Mergers and acquisitions are a common practice in the business world, where two companies combine their resources, expertise, and assets to form a new entity. In the case of Allergan’s acquisition by AbbVie, this strategic move was designed to enhance the pharmaceutical giant’s position in the market, expand its product portfolio, and create significant synergies.
The deal, announced in 2014, marked a significant milestone in the history of Botox, the world-renowned neurotoxin used for various medical and cosmetic applications. As part of the agreement, AbbVie acquired Allergan’s remaining 92% stake in Botox, gaining control over this highly profitable product.
Regulatory bodies, such as the US Food and Drug Administration (FDA), played a crucial role in shaping the landscape for M&A transactions involving pharmaceutical companies like Allergan and AbbVie. The FDA’s approval process for new drugs and therapies provides a critical safeguard against adverse effects, ensuring public safety and efficacy standards are met.
As part of its regulatory obligations, Abbott Laboratories (later renamed AbbVie) must adhere to strict guidelines set forth by the FDA. These regulations dictate the development, testing, and distribution of pharmaceutical products, including Botox. Compliance with these rules ensures that patients receive high-quality treatments while minimizing risks associated with their use.
Another key aspect to consider is antitrust law, which governs mergers and acquisitions in the United States. This body of legislation aims to promote competition by preventing monopolies and other anti-competitive practices. In the case of Allergan’s acquisition by AbbVie, regulatory scrutiny focused on potential concerns related to market dominance and impact on competition.
The history of Botox, from its inception in the 1960s as a treatment for facial spasms, has been shaped by an intricate dance between medical innovation, commercialization, and regulation. The acquisition of Allergan’s stake in Botox marked a new chapter in this narrative, solidifying AbbVie’s position as a major player in the global pharmaceutical industry.
From a business perspective, the deal provided opportunities for synergy creation, cost savings, and revenue growth through optimized distribution channels and expanded product portfolios. By consolidating Allergan’s 15-year-old partnership with Galderma, a leading skincare company, AbbVie was able to strengthen its global presence in dermatology.
However, the acquisition also faced opposition from stakeholders, including investors and customers. Critics argued that the deal would stifle innovation and limit access to Botox for patients who could not afford it or prefer alternative treatments. As a result, regulatory bodies and industry associations closely monitored the impact of the merger on competition and patient outcomes.
In conclusion, Allergan’s acquisition by AbbVie was a landmark deal that reflects the complex interplay between M&A strategies, regulations, and market dynamics in the pharmaceutical industry. By examining the historical context of Botox and its regulatory landscape, we gain insight into how business decisions shape the lives of consumers worldwide.
The history of *_Botox_* is a fascinating tale that spans decades, from its initial discovery in the 1960s to its current status as a beauty must-have.
It all began in the 1960s when *_Dr. Alan Scott_* discovered the potent neurotoxin, *_botulinum toxin_*, while working at the University of Pennsylvania’s *_Wistar Institute_*. The toxin was initially used to treat eye muscle spasms and other medical conditions.
However, it wasn’t until 2001 that a significant turning point occurred in *_Botox_*’s history. That year, the pharmaceutical company *_Allergan_* acquired the rights to *_botulinum toxin_* from the *_Dow Chemical Company_*. This marked a major milestone for *_Botox_* as it brought the treatment into mainstream use.
The acquisition by *_Allergan_* was instrumental in making *_Botox_* more accessible to a wider audience. The company began marketing *_Botox_* as a cosmetic treatment, and its popularity quickly soared.
One of the key factors that contributed to *_Botox_*’s success was the development of new applications for the toxin. Researchers began studying its effects on facial muscles, and soon, it became clear that *_Botox_* could be used to reduce wrinkles and fine lines.
The FDA approved *_Botox_* as a cosmetic treatment in 2002, paving the way for its widespread use. The agency granted *_Allergan_* permission to market *_Botox_* for the treatment of facial wrinkles and creases.
Despite the growing popularity of *_Botox_*, there were still concerns about its safety and regulation. In 2007, the FDA implemented new regulations to ensure that *_Botox_* was administered in a safe and controlled manner.
Today, *_Botox_* is one of the most popular cosmetic treatments in the world. It has been used by millions of people to reduce wrinkles and fine lines, and it continues to be a leader in the field of medical innovation.
In addition to its use as a beauty treatment, *_Botox_* also has a range of medical applications. It is used to treat conditions such as eyelid spasms, migraines, and excessive sweating.
The success of *_Botox_* can be attributed to the efforts of numerous researchers, scientists, and healthcare professionals who have worked tirelessly to advance our understanding of this complex neurotoxin.
As we look to the future, it is clear that *_Botox_* will continue to play an important role in shaping the world of medicine and beauty.
The process of a merger involves the combination of two or more companies to form a new entity, with the aim of increasing efficiency, reducing costs, and improving competitiveness.
Regulatory approval is a critical component of mergers, as it ensures that the combined company complies with relevant laws and regulations.
In many cases, regulatory approval requires the merged company to submit a detailed plan outlining how it will address any potential anticompetitive effects or other concerns raised by regulators.
The regulatory landscape for mergers can vary significantly depending on the jurisdiction and the type of business involved.
For example, in the United States, the Federal Trade Commission (FTC) is responsible for reviewing proposed mergers to ensure that they do not substantially lessen competition or create a monopoly.
In Europe, the European Commission has similar responsibilities, but its review process tends to focus more on the potential impact of a merger on trade and competition within the Single Market.
Regulatory approval can be obtained through a variety of means, including a formal investigation and negotiation with regulators, or through a simplified procedure that allows for faster review.
However, regulatory approval is not always guaranteed, and companies may need to make concessions or modifications to their business plans in order to secure approval.
In addition to addressing anticompetitive concerns, regulatory approval may also require companies to implement certain safeguards or reporting requirements to ensure that the merged entity remains transparent and accountable.
Regulatory approval can have a significant impact on the success of a merger, as companies that fail to obtain necessary approvals risk facing penalties or even having their merger declared invalid.
The regulatory environment for mergers is constantly evolving, with new regulations and laws being implemented in response to changing market conditions and societal values.
As a result, companies involved in mergers must stay informed about the latest developments and adapt their strategies accordingly in order to navigate the complex regulatory landscape successfully.
In the case of Botox, which has undergone significant transformations from its origins as a prescription-only medication to a widely used cosmetic treatment, regulatory approval has played a critical role in shaping its development and marketing.
The FDA’s initial concerns about the potential risks associated with botulinum toxin injections led to strict regulations surrounding its use, including requirements for rigorous testing and approval procedures.
However, as Botox became increasingly popular and its benefits were widely recognized, regulatory authorities began to relax their oversight, allowing the company to expand its marketing reach and make further modifications to its product.
Today, Botox is widely used in both medical and aesthetic contexts, with a range of approved indications for everything from facial wrinkles to muscle spasms, demonstrating the complex interplay between regulatory approval and market demand.
The history of Botox serves as a reminder that regulatory approval is an ongoing process, requiring companies to balance their pursuit of innovation and growth with the need for rigorous testing, safety, and efficacy standards.
The approval of Botox for the treatment of facial wrinkles and frown lines between the eyebrows marked a significant milestone in the history of the popular cosmetic treatment.
Get Your Dermal Filler Consultation with Dr. Laura Geige at It’s Me and You Clinic
As _Botox_ is a prescription-only medication, its approval by regulatory bodies such as the US FDA and EU indicates that it has undergone rigorous testing to demonstrate its safety and efficacy for this specific use case.
The FDA’s approval process for Botox involved extensive clinical trials to assess its ability to temporarily relax facial muscles and reduce wrinkles. These trials demonstrated that Botox is effective in reducing the appearance of moderate to severe frown lines between the eyebrows, which are a common symptom of _Botulinum Toxin_ related conditions.
Once approved, Botox manufacturers could begin marketing their product for this specific indication, and it quickly became a popular treatment for cosmetic concerns. The EU’s approval shortly after the FDA’s decision helped to further establish Botox as a widely accepted solution for facial wrinkles.
From a regulatory perspective, the approval of Botox highlights the complex interplay between _Medical Innovation_ and _Regulatory Agencies_. While the FDA and other regulatory bodies play a crucial role in ensuring the safety and efficacy of new treatments, they must also balance this with the need to facilitate innovation and progress in medical research.
The EU’s approval process for Botox, like that of many other cosmetic treatments, is governed by _European Cosmetics Regulation_ (ECR) and its various directives. The ECR establishes a framework for the safe use of cosmetics, including those containing _Botulinum Toxin_, while also recognizing the need for innovation and creativity in the field.
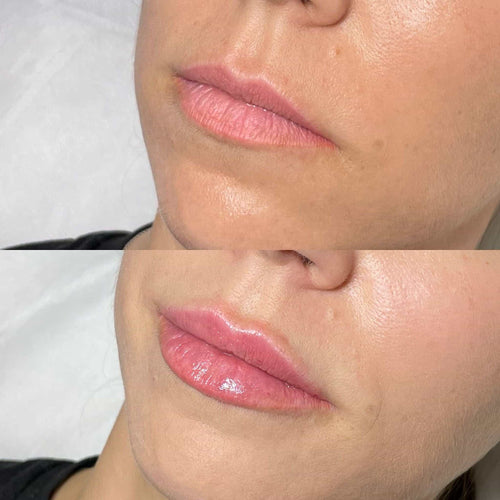
The approval of Botox for cosmetic use marked an important turning point in the history of the treatment, as it helped to establish its legitimacy as a popular cosmetic procedure. Today, Botox is one of the most widely used _Botulinum Toxin_ based treatments globally, with millions of injections performed each year.
The regulatory framework surrounding Botox has continued to evolve over time, with updates and revisions aimed at ensuring that the treatment remains safe and effective for its intended use. For example, the FDA has implemented strict guidelines for the use of Botox in certain _Cosmetic Procedures_ , such as the off-label use of the treatment for treating migraines.
In addition to these regulatory efforts, there are ongoing debates about the need for greater regulation of the cosmetic industry as a whole. Critics argue that inadequate regulations can lead to exploitation and misuse of treatments like Botox, while proponents contend that such regulations stifle innovation and limit access to effective treatments.
Ultimately, the history of Botox highlights the complex interplay between _Medical Innovation_ , regulatory frameworks, and public demand for effective treatments. As new treatments emerge and evolve, it is essential to strike a balance between these competing interests in order to ensure that patients have access to safe and effective solutions.
The future of Botox and other cosmetic treatments will likely be shaped by ongoing debates about regulation, innovation, and patient safety. One thing is clear, however: _Botox_ has become an integral part of modern cosmetic practice, and its impact on the field of dermatology and aesthetics will only continue to grow.
Cosmetic Use and Controversy
The history of cosmetic use dates back thousands of years, with ancient civilizations such as Egypt and Greece using various substances to alter their appearance.
In the early 20th century, the use of cosmetics was primarily limited to theatrical makeup and was considered taboo in everyday life.
However, with the rise of Hollywood’s Golden Age, the beauty industry began to boom, and cosmetic companies started to market products as “cosmetics” rather than just medicinal tonics.
In the 1950s and 60s, women like Elizabeth Taylor, Marilyn Monroe, and Jacqueline Kennedy became iconic beauty standards, driving the demand for wrinkle creams and other anti-aging products.
The 1970s saw a surge in popularity of makeup and hair styling products, with the rise of the disco era and the emergence of supermodels like Farrah Fawcett and Cher.
However, it wasn’t until the 1980s that cosmetic use truly became mainstream, with the introduction of popular beauty products like Estee Lauder’s “Youth Dew” and Revlon’s “Charisma” mascara.
The 1990s saw a significant increase in the popularity of Botox, as it began to be used for non-medical purposes such as wrinkle reduction and facial relaxation.
The controversy surrounding cosmetic use has long been present, with many arguing that the industry preys on vulnerable individuals and perpetuates unrealistic beauty standards.
Many celebrities have been vocal about their experiences with plastic surgery and Botox, further fueling the public’s perception of cosmetic use as a symbol of vanity and superficiality.
Despite this, there is also a growing movement to promote body positivity and self-acceptance, encouraging individuals to focus on inner beauty rather than physical appearance.
In recent years, there has been increased scrutiny of the cosmetic industry, with concerns over ingredients, animal testing, and environmental impact leading to greater awareness and regulation.
Many countries have introduced laws governing the use of certain ingredients, such as parabens and phthalates, while some companies are now opting for more natural and sustainable alternatives.
The rise of social media has also played a significant role in shaping public perception of cosmetic use, with influencers and celebrities sharing their personal beauty routines and experiences, both pro and anti.
Today, the cosmetics industry is a multibillion-dollar market, with new products and technologies emerging all the time, from LED face masks to virtual try-on apps.
As consumers become increasingly health-conscious and environmentally aware, the cosmetic industry must adapt to changing expectations and values, prioritizing both aesthetics and well-being.
Ultimately, the controversy surrounding cosmetic use will only continue to intensify as our understanding of beauty, wellness, and sustainability evolves.
Botox, a neurotoxin medication initially used to treat certain medical conditions, has been a cornerstone of beauty routines for over two decades.
The mid-2000s marked a significant turning point in the history of Botox, as its popularity skyrocketed with the help of celebrity endorsements and the widespread adoption of social media.
Celebrities like Jennifer Lopez, Gwyneth Paltrow, and Sharon Stone were among the first to popularize Botox, showcasing their smooth, youthful skin on the red carpet and in magazine covers.
The widespread use of social media platforms like Facebook, Twitter, and Instagram further amplified the beauty benefits of Botox, as users shared before-and-after photos and personal experiences with the treatment.
As a result, Botox became a status symbol, with many people seeking out the treatment to appear more youthful and attractive.
However, this newfound popularity also led to controversy surrounding the use of Botox in non-medical contexts.
Critics argued that the widespread promotion of Botox as a beauty solution was contributing to unrealistic expectations about appearance and perpetuating the stigma around aging.
Some medical professionals expressed concerns about the lack of regulation in the industry, with some clinics offering unproven or unauthorized treatments.
In response to these concerns, regulatory bodies began to take action, with the FDA imposing stricter guidelines on the marketing and distribution of Botox for cosmetic use.
The FDA required manufacturers to provide clearer warnings about potential side effects, such as drooping eyelids and facial asymmetry, and prohibited the use of unsubstantiated claims about its benefits.
Additionally, some countries implemented stricter regulations on the sale and use of Botox for non-medical purposes, such as requiring prescriptive authority or imposing age restrictions.
The controversy surrounding Botox highlights the complex relationship between beauty, technology, and medicine.
On the other hand, the commercialization of these technologies has also raised concerns about their impact on society and individual well-being.
The debate surrounding Botox continues to this day, with ongoing discussions about its use in cosmetics, the ethics of advertising it as a beauty treatment, and the need for greater regulation in the industry.
In conclusion, while Botox has undoubtedly become a staple in beauty routines worldwide, its widespread popularity also raises important questions about the intersection of technology, medicine, and aesthetics.
The history of Botox is a fascinating story that highlights the complex relationship between cosmetic use, controversy, criticism, and concern.
Botox, a neurotoxin derived from the bacterium Clostridium botulinum, has been used for over two decades to treat various medical conditions such as migraines, eyelid spasms, and excessive sweating. However, its popularity in cosmetic procedures has led to widespread debate about its use in beauty treatments.
Concerns about Cosmetic Use
The most significant concern surrounding Botox’s cosmetic use is its potential impact on facial muscles and overall health. Critics argue that frequent use can lead to a “frozen face,” causing a range of issues including:
-
Droopy eyelids (ptosis)
-
Nasal deformity
-
Weak facial expressions
-
Uneven eyebrows
-
A “mask-like” appearance
Additionally, the long-term effects of Botox on the body are not yet fully understood. Some research suggests that repeated injections may lead to changes in facial anatomy, such as a reduced nasolabial fold or an altered orbital rim.
Criticisms from Medical Professionals
Many medical professionals have expressed concerns about the lack of regulation in the cosmetic use of Botox. Without proper training and oversight, estheticians and non-medical practitioners may be injecting patients with improper doses, leading to adverse reactions and long-term damage.
Arrange a Consultation for Dermal Fillers with Dr. Laura Geige
-
Lack of standardization in dosing and technique
-
Insufficient education on proper injection techniques and after-care
-
Inadequate regulation of Botox distributors and retailers
Criticisms from Patient Advocacy Groups
Some patient advocacy groups have spoken out against the widespread use of Botox in cosmetic procedures, citing concerns about:
-
The emphasis on youth and beauty over individuality and self-acceptance
-
The lack of transparency about ingredient ingredients and potential side effects
-
Marketing and advertising practices that create unrealistic expectations and pressure patients to undergo procedures
Regulatory Challenges
The FDA has approved Botox for cosmetic use in 2002, but some critics argue that the approval process was flawed. The agency did not require clinical trials specifically designed to study Botox’s effects on facial muscles or overall health.
-
Lack of comprehensive premarket approval data
-
Insufficient long-term follow-up studies
-
Inadequate post-market surveillance and monitoring
The Future of Botox
As the popularity of cosmetic procedures continues to grow, it is essential that regulatory bodies, medical professionals, and patient advocacy groups work together to address these concerns.
-
Improved education and training for estheticians and practitioners
-
Standardization of dosing and technique guidelines
-
Enhanced transparency about ingredients, side effects, and potential long-term consequences
The history of Botox serves as a cautionary tale about the complex interplay between medical innovation, cosmetic use, and societal values. As we move forward, it is crucial that we prioritize patient safety, transparency, and individual autonomy in the pursuit of beauty and wellness.
The use of cosmetic products has been a contentious issue for many years, with concerns over their safety, efficacy, and environmental impact sparking heated debates among health experts and government agencies.
One product that has been at the forefront of these debates is *Botox*, a neurotoxin derived from the bacterium Clostridium botulinum that has become a staple in many beauty routines.
Botox was first introduced as a medical treatment for facial wrinkles and spasms in the 1980s, but its cosmetic use soon followed, with millions of people around the world turning to it to smooth out their wrinkles and fine lines.
However, despite its widespread use, concerns over safety have been raised. Some studies have suggested that *Botox* can cause a range of side effects, including eyelid drooping, facial asymmetry, and difficulty swallowing.
Moreover, there are also concerns over the efficacy of *Botox*. While it may provide short-term benefits, some experts argue that its effects are not long-lasting enough to be considered a reliable solution for cosmetic use.
Furthermore, the environmental impact of *Botox* is a major concern. The EPA has warned that the product can contaminate soil and waterways if not disposed of properly.
This highlights the need for more research into the safety and efficacy of cosmetic products like *Botox*, as well as greater regulation over their environmental impact.
Some experts argue that the beauty industry is driven by a “beauty industrial complex” that prioritizes profits over people’s health and the environment.
This has led to calls for greater transparency and accountability in the cosmetic industry, including mandatory labeling of ingredients and clearer warning labels about potential side effects.
Others are pushing for more sustainable alternatives to *Botox*, such as natural wrinkle treatments and botulinum-free creams.
The debate over the safety, efficacy, and environmental impact of cosmetic products like *Botox* highlights the need for greater awareness and regulation in the industry.
The cosmetic use of Botox has become a ubiquitous practice in modern beauty standards, with millions of people around the world seeking to reduce wrinkles and fine lines on their faces.
However, the history of Botox is complex and multifaceted, spanning over three decades as a medical treatment for various conditions. In its early days, Botox was first discovered in 1900 by German researcher Oskar Alfred Groth, who isolated the toxin from a bacterium that causes botulism.
Initially, Botox was used to treat crossed eyes and other neurological disorders, such as muscle spasms and migraines. In the 1980s, a team of researchers at Allergan discovered the ability to use Botox for cosmetic purposes, specifically to temporarily relax facial muscles that cause wrinkles.
One of the key figures behind the development of Botox for cosmetics was Jean Carruthers, an American dermatologist who was one of the first to recognize its potential. In 1989, Carruthers and her husband, a plastic surgeon, began using Botox to treat patients with facial wrinkles, leading to significant reductions in fine lines and wrinkles.
The FDA approved Botox for cosmetic use in 2002, paving the way for its widespread adoption in the beauty industry. Since then, Botox has become a staple of modern beauty treatments, with millions of people seeking regular injections to maintain smooth, wrinkle-free skin.
Despite its popularity, however, Botox has also been surrounded by controversy and debate. Critics argue that its cosmetic use is unnecessary and promotes unrealistic beauty standards, perpetuating the notion that a smooth face is essential for attractiveness.
Some have also raised concerns about the long-term safety effects of repeated Botox injections, particularly with regards to muscle atrophy, eyelid drooping, and other potential side effects.
Additionally, there has been controversy surrounding the business practices of companies that market Botox for cosmetics, including allegations of exaggerating its benefits and making false claims about its effectiveness.
Furthermore, the lack of regulation in the beauty industry has led to concerns about the quality and safety of Botox being administered by unlicensed practitioners. This has resulted in several high-profile cases of botched treatments and serious complications.
In response to these concerns, regulatory agencies such as the FDA have implemented stricter guidelines for the use and marketing of Botox, as well as increased inspections and enforcement measures.
Despite these efforts, however, the cosmetic use of Botox remains a contentious issue. As the industry continues to evolve, it is likely that we will see further debates about its safety, efficacy, and cultural significance.
In recent years, there has been a growing movement towards more natural and holistic approaches to beauty, with many people seeking alternatives to Botox and other cosmetic treatments. This shift has led to increased interest in non-surgical treatments such as facials, chemical peels, and microneedling.
At the same time, some companies are pushing back against what they see as an overemphasis on natural beauty, arguing that Botox and other cosmetic treatments are necessary for maintaining confidence and self-esteem.
The debate surrounding Botox reflects a broader cultural shift towards reevaluating our attitudes towards beauty and aging. As we become increasingly aware of the environmental and social impacts of our choices, it is likely that our approaches to beauty will continue to evolve in response.
Botox has become an integral part of modern beauty standards, with millions of people worldwide relying on it to achieve a smoother, more youthful appearance.
But beyond its aesthetic benefits, Botox has also been surrounded by controversy and debate.
One of the main concerns surrounding Botox is its impact on facial muscles and the potential for dependency.
When used excessively or in incorrect doses, Botox can cause a range of side effects, including droopy eyelids, uneven eyebrows, and difficulty smiling.
Furthermore, there have been reports of Botox being misused by individuals seeking to conceal signs of anxiety or other emotional distress.
This has led some experts to warn that the use of Botox as a beauty treatment can perpetuate negative attitudes towards mental health.
Another concern is the lack of regulation in the cosmetic industry, which allows unqualified practitioners to administer Botox treatments without proper training or oversight.
This can lead to serious complications, including botulism, a potentially life-threatening condition that occurs when the toxin spreads beyond the intended treatment area.
Environmental concerns have also been raised about the use of Botox, with some experts arguing that it contributes to the growing problem of plastic waste and the exploitation of animals in the cosmetics industry.
Botox is produced from a bacterium called Clostridium botulinum, which can be found naturally in soil and water.
However, most commercial Botox products are grown using microorganisms that have been genetically engineered to produce larger quantities of the toxin.
This has raised concerns about the potential for contamination and the impact on the environment.
To address these concerns, some companies are now developing more sustainable alternatives to traditional Botox, such as botulinum toxin-based serums that use plant-derived ingredients or lab-grown microorganisms.
Ongoing research is also focusing on improving the safety, efficacy, and sustainability of Botox treatments.
For example, studies are exploring the use of lower doses and more targeted application methods to minimize side effects while maintaining effectiveness.
Additionally, researchers are investigating new delivery systems, such as nanoparticles and biodegradable gels, that could improve the safety and convenience of Botox injections.
As the cosmetics industry continues to grow and evolve, it’s essential to address these concerns and prioritize both human health and environmental sustainability.
Ultimately, the future of cosmetic treatments like Botox will depend on our ability to strike a balance between innovation, safety, and responsibility.
Read more about James Martin Live here. Read more about Alkhemist LA here. Read more about Detailed Weddings LA here. Read more about Fringe Beverly Hills here.
- Do And Don’ts After Face Fillers? - May 8, 2025
- Non Surgical Butt Lift In Croydon Surrey - May 8, 2025
- Neck Line Filler Treatment Near Banstead, Surrey - May 7, 2025
